Products
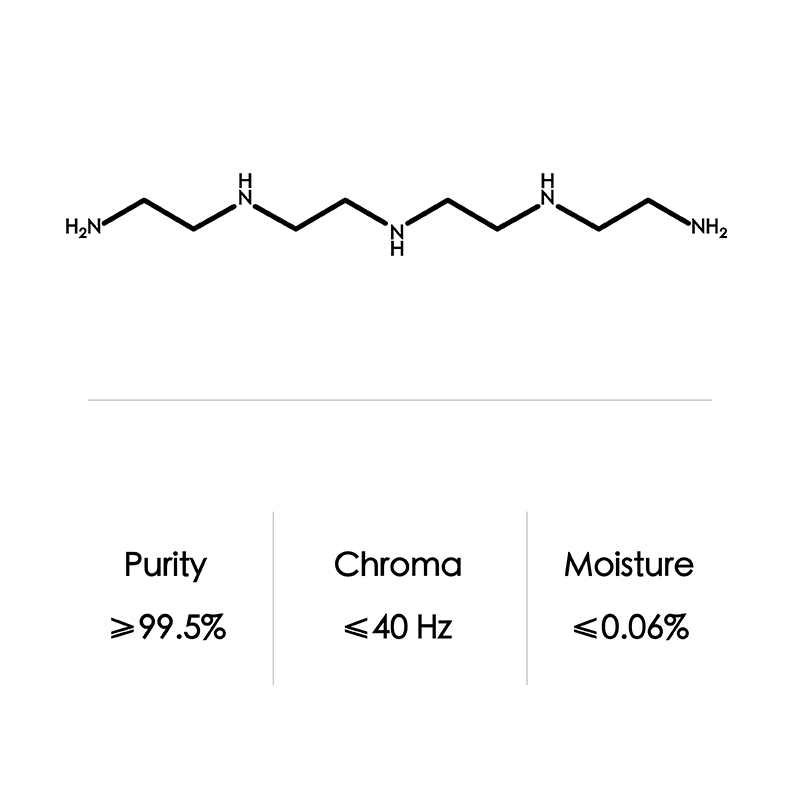
Tetraethylenepentamine CAS No. 112-57-2
Product Description
Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encountered as a colorless, viscous liquid. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans".
Properties
| Formula | C8H23N5 | |
| CAS NO | 112-57-2 | |
| appearance | colorless, transparent, viscous liquid | |
| density | 0.998 g/cm³ | |
| boiling point | 340 ℃ | |
| flash(ing) point | 139℃ | |
| packaging | drum/ISO Tank | |
| Storage | Store in a cool, ventilated, dry place, isolated from the fire source, loading and unloading transportation should be stored in accordance with the provisions of flammable toxic chemicals | |
*The parameters are for reference only. For details, refer to COA
Application
| Mainly used in the synthesis of polyamide resin, cation exchange resin, lubricating oil additives, fuel oil additives, etc., can also be used as epoxy resin curing agent, rubber vulcanization accelerator. |
Diethanolamine is used in metalworking fluids for cutting, stamping and die-casting operations as a corrosion inhibitor. In the production of detergents, cleaners, fabric solvents and metalworking fluids, diethanolamine is used for acid neutralization and soil deposition. DEA is a potential skin irritant in workers sensitized by exposure to water-based metalworking fluids. One study showed that DEA inhibits in baby mice the absorption of choline, which is necessary for brain development and maintenance;[8] however, a study in humans determined that dermal treatment for 1 month with a commercially available skin lotion containing DEA resulted in DEA levels that were "far below those concentrations associated with perturbed brain development in the mouse".In a mouse study of chronic exposure to inhaled DEA at high concentrations (above 150 mg/m3), DEA was found to induce body and organ weight changes, clinical and histopathological changes, indicative of mild blood, liver, kidney and testicular systemic toxicity.
DEA is a potential skin irritant in workers sensitized by exposure to water-based metalworking fluids.One study showed that DEA inhibits in baby mice the absorption of choline, which is necessary for brain development and maintenance;[8] however, a study in humans determined that dermal treatment for 1 month with a commercially available skin lotion containing DEA resulted in DEA levels that were "far below those concentrations associated with perturbed brain development in the mouse". In a mouse study of chronic exposure to inhaled DEA at high concentrations (above 150 mg/m3), DEA was found to induce body and organ weight changes, clinical and histopathological changes, indicative of mild blood, liver, kidney and testicular systemic toxicity.A 2009 study found that DEA has potential acute, chronic and subchronic toxicity properties for aquatic species
Advantage
Product quality, sufficient quantity, effective delivery, high quality of service It has an advantage over a similar amine, ethanolamine, in that a higher concentration may be used for the same corrosion potential. This allows refiners to scrub hydrogen sulfide at a lower circulating amine rate with less overall energy usage.