Products
Organic Chemicals Solvent Diethylene Glycol Product CAS No 111-46-6 Transparent Liquid
Product Description
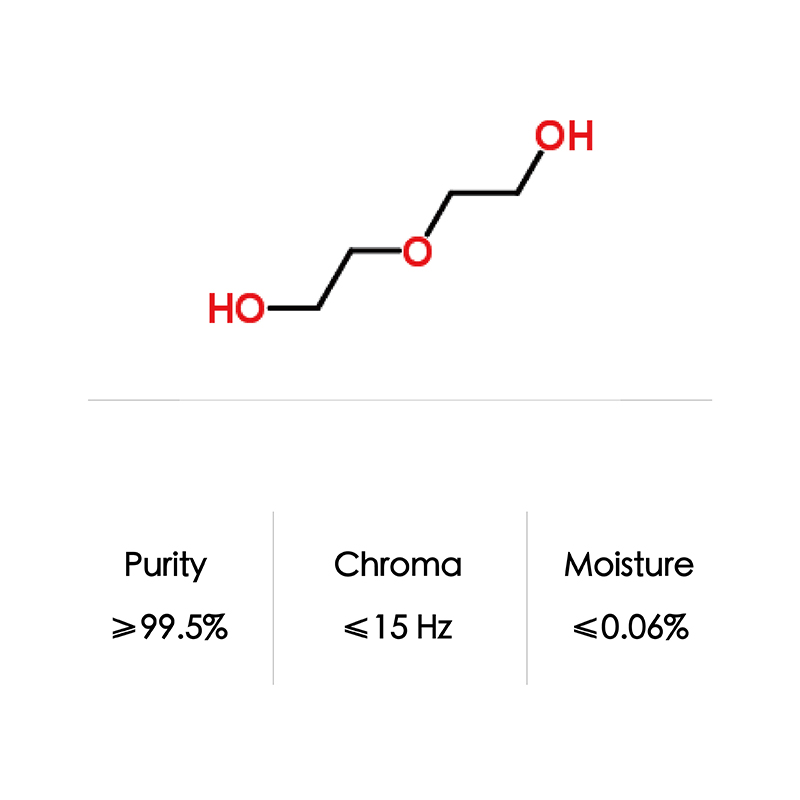
DEG is produced by the partial hydrolysis of ethylene oxide. Depending on the conditions, varying amounts of DEG and related glycols are produced. The resulting product is two ethylene glycol molecules joined by an ether bond.
"Diethylene glycol is derived as a co-product with ethylene glycol (MEG) and triethylene glycol. The industry generally operates to maximize MEG production. Ethylene glycol is by far the largest volume of the glycol products in a variety of applications. Availability of DEG will depend on demand for derivatives of the primary product, ethylene glycol, rather than on DEG market requirements."
Properties
| Formula | C4H10O3 | |
| CAS NO | 111-46-6 | |
| appearance | colorless, transparent, viscous liquid | |
| density | 1.1±0.1 g/cm3 | |
| boiling point | 245.7±0.0 °C at 760 mmHg | |
| flash(ing) point | 143.3±0.0 °C | |
| packaging | drum/ISO Tank | |
| Storage | Store in a cool, ventilated, dry place, isolated from the fire source, loading and unloading transportation should be stored in accordance with the provisions of flammable toxic chemicals | |
*The parameters are for reference only. For details, refer to COA
Application
| Used as gas dehydrating agent and aromatics extraction solvent, also used as textile lubricant, softener and finishing agent, as well as plasticizer, humidifier, sizing agent, nitrocellulose, resin and grease solvent. |
Diethylene glycol is used in the manufacture of saturated and unsaturated polyester resins, polyurethanes, and plasticizers.DEG is used as a building block in organic synthesis, e.g. of morpholine and 1,4-dioxane. It is a solvent for nitrocellulose, resins, dyes, oils, and other organic compounds. It is a humectant for tobacco, cork, printing ink, and glue.It is also a component in brake fluid, lubricants, wallpaper strippers, artificial fog and haze solutions, and heating/cooking fuel.In personal care products (e.g. skin cream and lotions, deodorants), DEG is often replaced by selected diethylene glycol ethers. A dilute solution of diethylene glycol can also be used as a cryoprotectant; however, ethylene glycol is much more commonly used. Most ethylene glycol antifreeze contains a few percent diethylene glycol, present as a byproduct of ethylene glycol production.
Advantage
Product quality, sufficient quantity, effective delivery, high quality of service It has an advantage over a similar amine, ethanolamine, in that a higher concentration may be used for the same corrosion potential. This allows refiners to scrub hydrogen sulfide at a lower circulating amine rate with less overall energy usage.