Products
High Quality Ethanolamine/ Monoethanolamine (MEA) CAS 141-43-5
Product Description
MEA can be produced by reacting ammonia/water with ethylene oxide at a pressure of 50–70 bar to keep ammonia in the liquid phase. The process is exothermic and does not require any catalyst. The ratio of ammonia and ethylene oxide plays a significant role in deciding the composition of the resulting mixture. If ammonia reacts with one mole of ethylene oxide, monoethanolamine is formed, with two molecules of ethylene oxide, diethanolamine is formed whereas with three moles of ethylene oxide triethanolamine is formed. After the reaction, the distillation of the resulting mixture is carried out first to remove excess ammonia and water. Then the amines are separated using a three-step distillation setup.
Properties
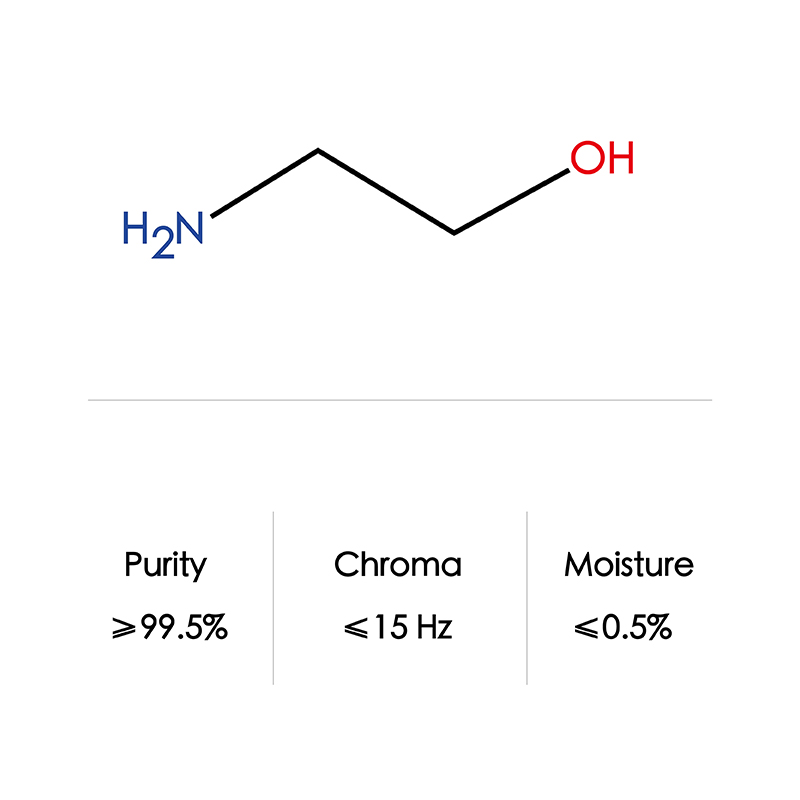
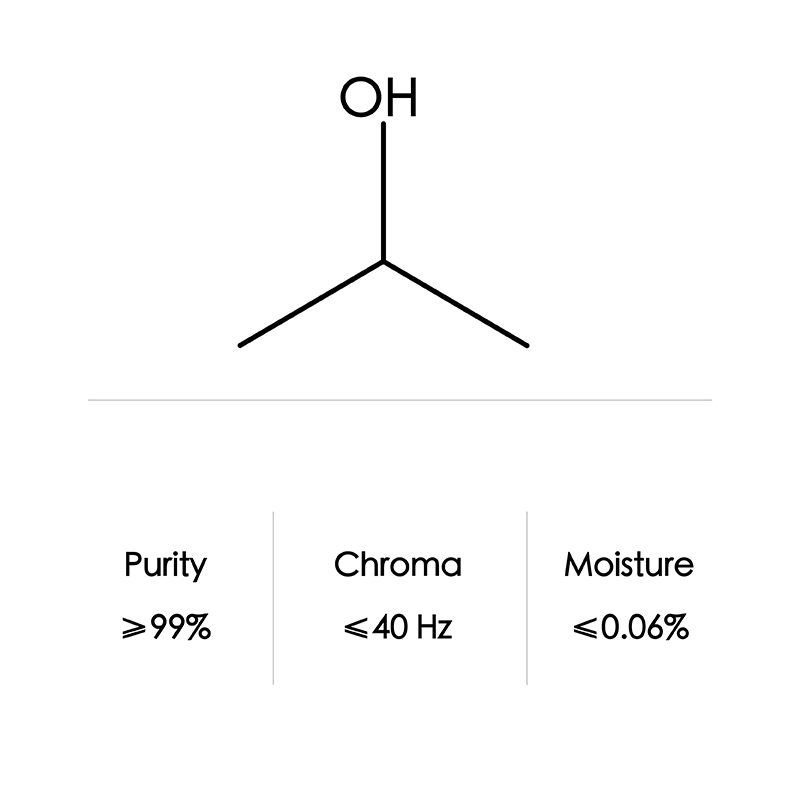
| Formula | C2H7NO | |
| CAS NO | 141-43-5 | |
| Appearance | colorless, transparent, viscous liquid | |
| Density | 1.02 g/cm³ | |
| Boiling point | 170.9 ℃ | |
| Flash(ing) point | 93.3 ℃ | |
| Packaging | 210 kg plastic drum/ISO Tank | |
| Storage | Store in a cool, ventilated, dry place, isolated from the fire source, loading and unloading transportation should be stored in accordance with the provisions of flammable toxic chemicals |
|
*The parameters are for reference only. For details, refer to COA
Application
| Chemical reagents, solvents, emulsifiers |
| Rubber accelerators, corrosion inhibitors, deactivators |
Monoethanolamine is used as chemical reagents, pesticides, medicines, solvents, dye intermediates, rubber accelerators, corrosion inhibitors and surfactants, etc. It is also used as acid gas absorbents, emulsifiers, plasticizers, rubber vulcanizing agents, printing and dyeing Whitening agent, fabric anti-moth agent, etc. It can also be used as a plasticizer, vulcanizing agent, accelerator and foaming agent for synthetic resins and rubber, as well as intermediates for pesticides, medicines and dyes. It is also a raw material for synthetic detergents, emulsifiers for cosmetics, etc. Textile industry as printing and dyeing brightener, antistatic agent, anti-moth agent, detergent. It can also be used as a carbon dioxide absorber, ink additive, and petroleum additive.
Advantage
Product quality, sufficient quantity, effective delivery, high quality of service It has an advantage over a similar amine, ethanolamine, in that a higher concentration may be used for the same corrosion potential. This allows refiners to scrub hydrogen sulfide at a lower circulating amine rate with less overall energy usage.