Products
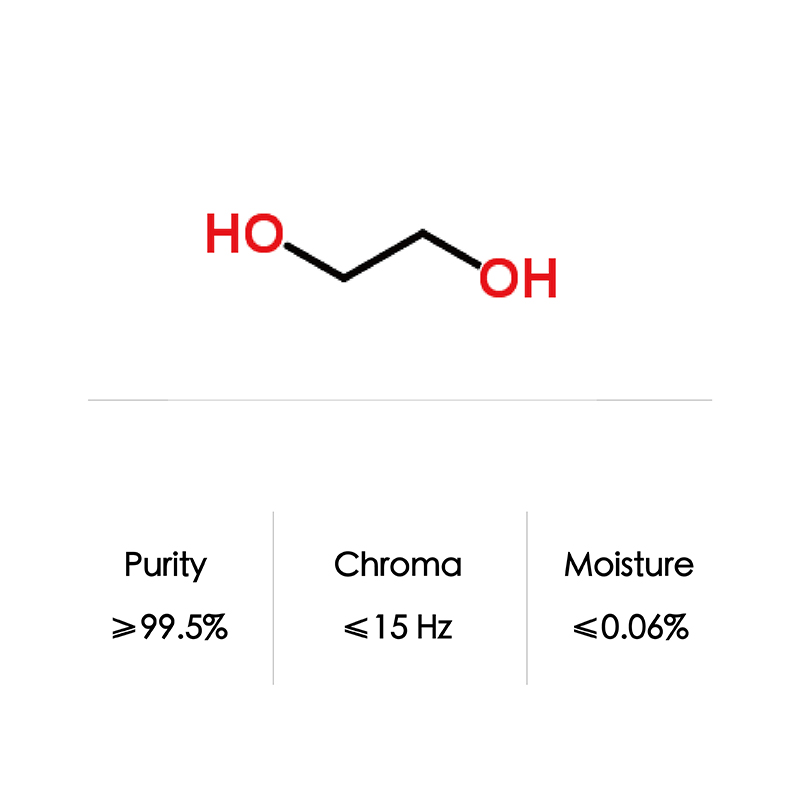
CAS No 107-21-1 Industrial Grade 99% Mono Ethylene Glycol
Product Description
The major use of ethylene glycol is as an antifreeze agent in the coolant in for example, automobiles and air-conditioning systems that either place the chiller or air handlers outside or must cool below the freezing temperature of water. In geothermal heating/cooling systems, ethylene glycol is the fluid that transports heat through the use of a geothermal heat pump. The ethylene glycol either gains energy from the source (lake, ocean, water well) or dissipates heat to the sink, depending on whether the system is being used for heating or cooling.
Pure ethylene glycol has a specific heat capacity about one half that of water. So, while providing freeze protection and an increased boiling point, ethylene glycol lowers the specific heat capacity of water mixtures relative to pure water. A 1:1 mix by mass has a specific heat capacity of about 3140 J/(kg·°C) (0.75 BTU/(lb·°F)), three quarters that of pure water, thus requiring increased flow rates in same-system comparisons with water.
Properties
| Formula | C2H6O2 | |
| CAS NO | 107-21-1 | |
| appearance | colorless, transparent, viscous liquid | |
| density | 1.1±0.1 g/cm3 | |
| boiling point | 197.5±0.0 °C at 760 mmHg | |
| flash(ing) point | 108.2±13.0 °C | |
| packaging | drum/ISO Tank | |
| Storage | Store in a cool, ventilated, dry place, isolated from the fire source, loading and unloading transportation should be stored in accordance with the provisions of flammable toxic chemicals | |
*The parameters are for reference only. For details, refer to COA
Application
| Mainly used for the production of synthetic resins, surfactants and explosives, but also used as antifreeze |
The mixture of ethylene glycol with water provides additional benefits to coolant and antifreeze solutions, such as preventing corrosion and acid degradation, as well as inhibiting the growth of most microbes and fungi.Mixtures of ethylene glycol and water are sometimes informally referred to in industry as glycol concentrates, compounds, mixtures, or solutions.
In the plastic industry, ethylene glycol is an important precursor to polyester fibers and resins. Polyethylene terephthalate, used to make plastic bottles for soft drinks, is prepared from ethylene glycol.
Advantage
Product quality, sufficient quantity, effective delivery, high quality of service It has an advantage over a similar amine, ethanolamine, in that a higher concentration may be used for the same corrosion potential. This allows refiners to scrub hydrogen sulfide at a lower circulating amine rate with less overall energy usage.